Interdisciplinary Note (17 of 17)
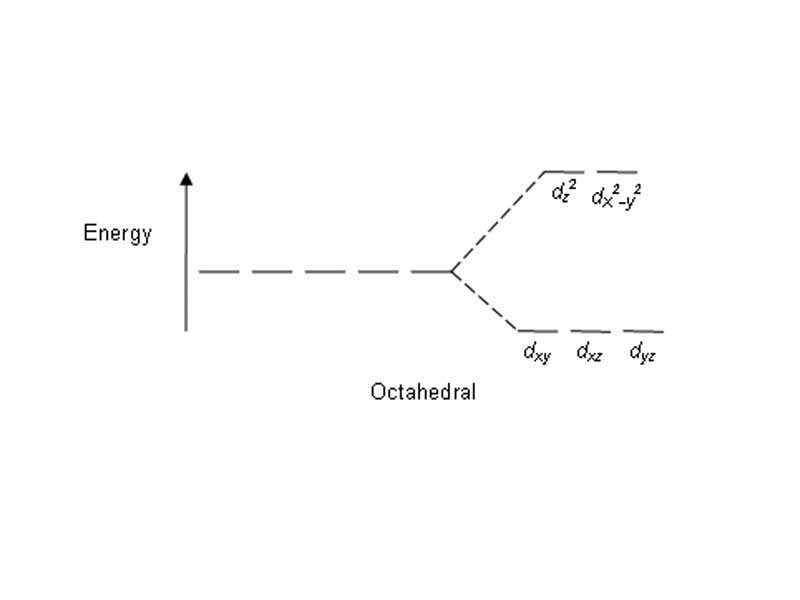
Many coordination complexes are brightly colored. The electron pairs brought to the complex by the ligands impinge on the d subshell electrons of the transition metal, pushing harder on those within two of the d subshell orbitals than those within the other three. This splits the d subshell electrons of the metals into two subsets, one whose energy is raised by impingement the ligand electrons more than the other. This is called crystal field splitting.
Because the d subshell electrons don't all have the same energy anymore, there is the possibility of absorption and electronic transition between the different energy states. crystal field splitting explains the colors of transition metal complexes,he energy of excitation between the two sets corresponding to photon energy in the visible range. When the complex absorbs a visible photon an electron transitions from the lower energy d orbitals to an excited state. Because only certain wavelengths are absorbed, the substance appears the complementary colour (if red is absorbed, green is observed).
WThis is why many minerals are brightly colored such as malachite, turquoise, citrine, emerald, ruby or amethyst, whether from crystalized coordination complexes or coordinated impurities.