Cross-Topic Integration (22 of 26)
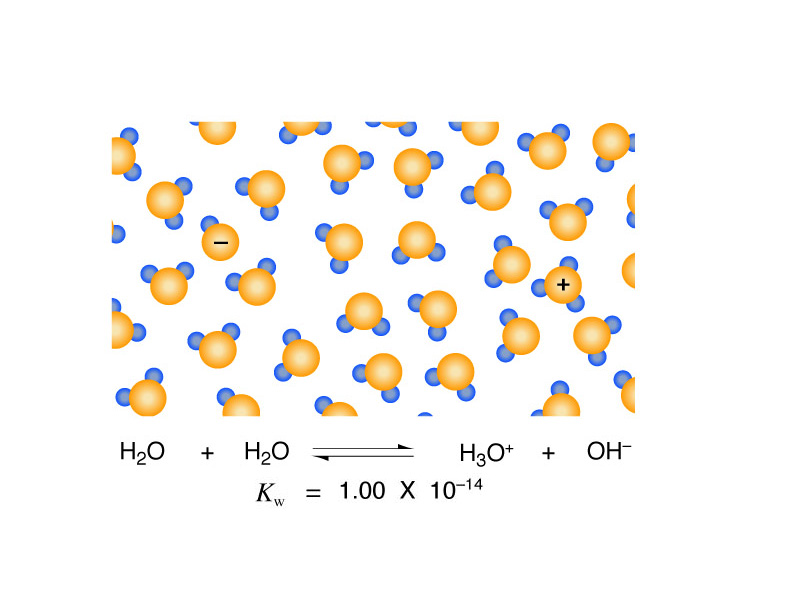
Water undergoes autoprotolysis. One water molecule will donate its proton to another water molecule forming a positive hydronium ion and a negative hydroxide ion. H+(aq) is used as an abbreviation for hydronium, and we are all in the habit of calling hydronium ions "hydrogen ions". Autoprotolysis achieves an equilibrium balance reflecting a dynamic process. Water is continuously dissociating into ions and returning to itself, and the ion product of water, a short-hand equilibrium constant, kw, describes the position of this equilibrium.
In solution chemistry, we introduced a similar short-hand equilibrium constant, the ksp, or solubility product, which describes the position of equilibrium in aqueous solution for the dissociation of a weak electrolyte, or in other words, a sparingly soluble salt, such as PbSO4. The salt dissociates into ions in dissolving in water. A saturated solution of a weak electrolyte is a dynamic process with the salt continuously dissociating into ions and returning to the undissolved material on the bottom of the beaker.
It can be useful to think of the autoprotolysis of water in similar terms to the dissociation of a salt, except that in recombining, the water doesn't fall to the bottom of the beaker, the ions just return to being water. Water is always "saturated" with its own ions. If you were to add NaSO4 to a saturated PbSO4 solution, precipitation would occur until the ion product in the solution (reaction quotient) finds itself again with the value of the solubility product (equilibrium constant). But now the balance will have shifted in the expression between the concentrations of Pb2+ and SO42-. Now the sulfate ion concentration is high and the lead ion concentration is tiny in the saturated solution. Of course, ksp has the same value as before, it reattained itself, though with the balance shifted between the Pb2+ and SO42-.
The autoprotolysis of water works the same way. A Brønsted acid added to water increases the concentration of hydrogen ions. In response, autoprotolysis runs in reverse to recapture the equilibrium described by kw, but with each hydrogen ion it takes up, it also takes up a hydroxide, reforming water, until the ion product of water is recovered. However, now the hydrogen ion concentration is higher than the hydroxide ion concentration. The pH is acidic.
In aqueous solution, the equilibrium between an acid and its conjugate base is always coupled to the autoprotolysis of water. A Brønsted acid or base introduced to the aqueous system establishes a coupled equilibrium with the autoprotolysis reaction. Water always intermediates, giving protons to the conjugate base and receiving protons from the acid. A Brønsted acid donates protons and, as such, will cause the balance to shift towards H+ within kw.