Interdisciplinary Note (12 of 17)
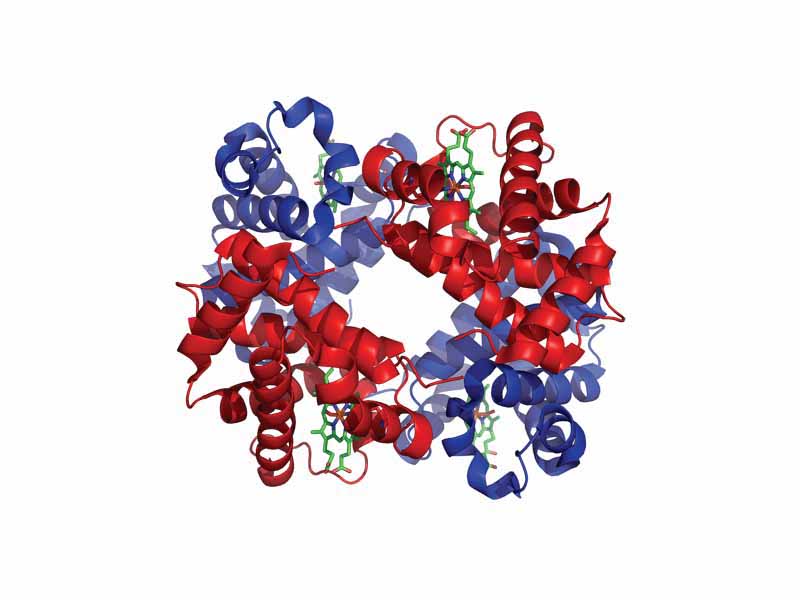
Many transport proteins bind small molecules and transport them to other locations in the body. These proteins typically have a high binding affinity when their ligand is present in high concentrations and a low binding affinity at the target tissues. Hemoglobin is the quintessential transport protein, which transports oxygen from the lungs to the tissues. The binding of oxygen by hemoglobin is a cooperative process. Binding affinity is increased by the degree of oxygen saturation, with the first oxygens bound causing steric changes that facilitate binding for the next oxygens. Furthermore, low pH and high CO2 levels in the tissues decrease binding capacity of hemoglobin for oxygen, a phenomenon known as the Bohr effect.
In allosteric interactions, the binding of a molecule to one site on the protein leads to conformational changes that affect a distant site on the protein. In hemoglobin, O2 binding is cooperative (the first binding enhances additional binding by the other heme groups nested in the other polypeptide chains). Cooperative binding in hemoglobin has found its way onto many MCATs. Additionally, the binding of H+ or CO2 allosterically alters the binding of O2. The allosteric changes in hemoglobin occur through subunit interaction. Most allosteric changes occur by means of quaternary structure. O2 binding relaxes the hemoglobin tetramer, promoting additional binding. The molecule BPG decreases affinity by stabilizing the deoxyhemoglobin quaternary structure. CO2 binding occurs at the terminal amino groups which take up carbamate to form salt bridges which stabilize deoxyhemoglobin. Furthermore, with deoxyhemoglobin, a certain histidine side chain gains affinity for H+, so acidic conditions stabilize deoxyhemoglobin.
These effects are summarized in the Bohr effect. An increase in blood carbon dioxide level, a decrease in pH or increase in temperature leads hemoglobin to bind oxygen less strongly. The Bohr effect supports the oxygen transport function of hemoglobin. Hemoglobin binds to oxygen in the lungs, but then releases it in the tissues, where carbon dioxide levels are high and pH is low.